A good aquarium filter will keep the water in the tank clean from some of the most toxic organic pollutants but it does not, however, keep the water as clean as it is in the rivers, lakes and oceans where your fish come from.
This is because the natural process that breaks down organic matter, called the nitrogen cycle, is incomplete in most aquarium environments.
Therefor, as organic waste from fish (feces, urine, etc.) and leftover food builds up in an aquarium over time, the water quality deteriorates.
There seems to be a varying degree of comprehension about this among aquarium hobbyists so here is an explanation of that deterioration.
Toxins
There are 3 toxins created by the breakdown of organic matter (fish waste, leftover food, etc.) in your aquarium:
1. Ammonia (also created by fish directly),
2. Nitrite, and
3. Nitrate.
In addition to those toxins, it is important to consider 2 other factors, Oxygen content and pH level(acidity).
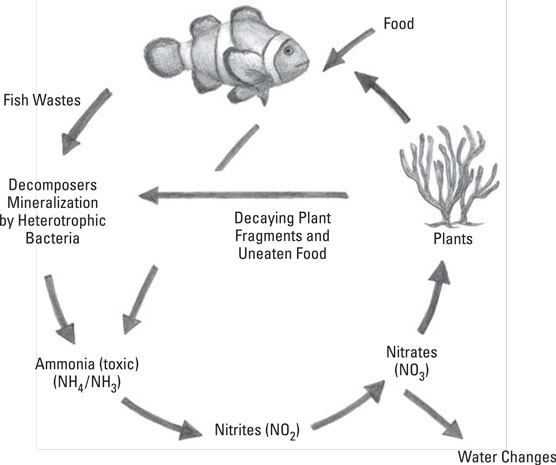
When organic matter falls to the bottom of the aquarium or gets caught in the filter, it is bacteria that do most of the work to clean it up. The bacteria eats the waste.
One type of bacteria eats the organic waste and creates its own waste in the form of Ammonia. Ammonia is highly toxic and lethal to fish and invertebrates.
Then a second type of bacteria, most commonly Nitrosomonas, begins eating the Ammonia and creates Nitrite as waste. Nitrite is also highly toxic and lethal to fish. A third type of bacteria, Nitrobacter, eats Nitrite and leaves Nitrate as waste.
Filtration
In under-filtered aquariums or if excessive amounts of organic matter are quickly added to the tank (i.e., adding too many fish at once or overfeeding), Ammonia can increase quickly and cause the bacteria to bloom explosively. It may even cause cloudiness in the water.
This can, in turn, use a tremendous amount of Oxygen.
When this happens, you will sometimes see the fish at the surface of the tank gasping for air. Ammonia also directly burns the gills and skin of the fish causing them stress which can lead to illness.
A similar result will happen if there is a Nitrite spike. When the Ammonia spikes, the decrease in Oxygen, combined with the acidic nature of Ammonia, will drop the pH to unacceptable levels.
An interesting thing happens here though because Ammonia is less toxic to fish in water with a low pH.
Stability
The problem with accepting this as a stable environment for fish to live in is that the Ammonia gets into the bloodstream of the animals in the aquarium.
Then, when a water change is done, the pH of the aquarium water is increased back to the appropriate level, making the Ammonia toxic again. The Ammonia in a fish’s bloodstream will then burn the fish from the inside causing internal damage.
Lastly, Nitrate presents another issue. It is less toxic than Ammonia or Nitrite but, in high levels, can become toxic to fish.
Under normal operating conditions, the bacteria will keep converting Ammonia to Nitrite and Nitrite to Nitrate, eliminating the most lethal of the 3 toxins.
However, the bacteria that breaks down Nitrate, turning it into gas to be expelled into the atmosphere, is extremely difficult to grow in an aquarium because it requires an oxygen free environment. This means that the Nitrate slowly builds up, over time, to levels that can stress and even kill fish.
The only method for managing Nitrate build-up in marine, fish-only (in other words, not reef) aquariums is dilution. In other words, frequent water changes.
Water changes and other regular maintenance can be tedious and time consuming but we are here to help. Book a consultation and we will set up a regular maintenance schedule tailored for your aquarium.
